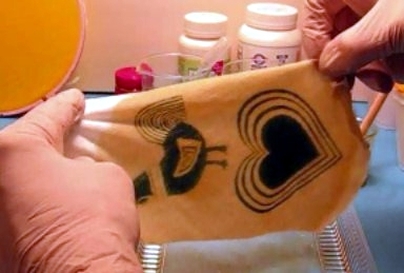
This post entry is based on my attempts to develop a photo bleachout system that is easy to use, educational, and fun. However, the final results so far have been a work in progress; but, there are some very interesting details discovered along the way that can become a good idea starter. So, I will share what I have done so far and hopefully others will help to add to the process disclosed. I am very well acquainted with failure and learn from each misadventure and eventually succeed.
This project is based on Turmeric, an Indian spice that has been used as a dye for over 2000 years. Sometimes spelled tumeric, it contains Curcumin, (3 to 5%), a polyphenol which reacts with boron under specific conditions to form a dye called rosocyanine. This test has been in use for about 100 years and the complex formed between the yellow Curcumin and the element boron forms the red or orange-red dye rosocyanine, which is a green crystal when isolated. This process has its early roots in 1842 when John Hershel began working with plants to form bleached out images. Called anthotypes, the process never really caught on as the images were generally of poor quality, took a long time to obtain, and did not last when exposed to light. But, all of the photosensitive materials were derived from plants. There is also evidence that turmeric was used and even an obscure article in Photophysics used a boric acid spray to develop the image. But, to my knowledge there has not been any attempt to enhance the image or make a permanent image as I am attempting to do with the sodium hydroxide and alum.
The materials used in this project are readily available locally either at the super market, hardware store, or garden center. The concentrations are low so the process is fairly safe. But, Turmeric is a strong dye and can stain whatever it comes in contact with so be prepared. The entire process and materials and processes will follow the video. If you watched the video on YouTube just skip to the second video and the instructions below:
First, prepare the Curcumin tincture from the turmeric (available in the spice section of most markets) by adding about 1 gram of turmeric to 100 milliliters of 91% isopropyl alcohol. Curcumin is not water soluble so will dissolve in the alcohol. The remainder of the turmeric will settle to the bottom and should be discarded. This can best be done by filtering through a coffee filter but can be simply decanted into a clean container and the sludge discarded. CAUTION: The Curcumin will stain any porous surface so work on a well protected surface.
Second, prepare a solution of boric acid by adding 4 grams of boric acid (ant and roach powder available at hardware and garden centers) to 196 milliliters of water. Stir until dissolved.
Third, prepare a solution of sodium hydroxide (lye or drain cleaner from the hardware store) with 4 grams sodium hydroxide to 196 milliliters of water. Stir until dissolved.
Fourth, prepare a solution of alum, potassium aluminum sulfate, (available in the spice section of the market or garden center as it is used in pickling food) with 4 grams alum to 196 milliliters of water.
Note: These are all 2% solutions, and are not critical, and close is close enough!

This is the light stability image after 2 weeks in the sunlight. Not too bad but still not completely permanent.
The fading of the final image in video 2 was in the sodium hydroxide solution for about 10 minutes while I changed SD cards. But, the image should be removed as soon as it is developed to avoid the fading.
I hope that you get involved and can add to the information and most of all, have fun! Ken.











 RSS Feed
RSS Feed
