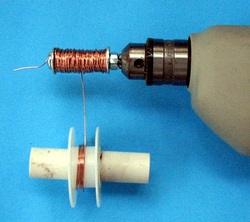
In the early 1900's, it was believed that ferromagnetic materials like iron, when placed in a coil of wire and subjected to a magnetic field, would orient the magnetic domains instantaneously. In 1919, Heinrich Barkhausen, while working in magnetism and acoustics, discovered that in fact, the orientation of the domains occurred in steps and in a discreet manner.
All ferromagnetic materials have domains that are, at the atomic level, are normally aligned in a straight line. When a magnetic field approaches, the domains align with the magnetic field.
This demonstration shows that effect and reproduces the results obtained by Barkhausen. If you need magnet wire to wind the coil for the experiment, Radio Shack has a three-pack of assorted gauges and lengths. But as you will see in the video, coil winding is not necessary. The links follow the video:
 RSS Feed
RSS Feed
