
I have had the opportunity to synthesize photochromics in a laboratory setting, and I have attempted to fabricate some with readily available materials at home but without success so far. But I will continue to try! But, for these experiments I bought Solar Drops ™ from a company called Solar Color Dust. Since I do not endorse products I have not included a link, but there are many products available from several companies. I purchased 6 bottles (six different colors) of 6ml each with approximately 100 drops per bottle. These are designed to be mixed with epoxy at the rate of 10 drops for each 1 ounce (28 grams) of resin. They were expensive at $20 plus shipping. I have included several photos with the results and some associated comments:

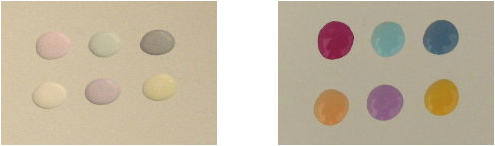
 RSS Feed
RSS Feed
