- Potassium Ferricyanide (10 grams dissolved in 100 ml distilled water) Sensitizer called part A.
- Ammonium Ferric Citrate Green Variety (25 grams dissolved in 100 ml distilled water) Sensitizer called part B.
- Mix part A with part B in equal amounts in subdued light. (About 15ml for an 8X10" sheet of paper).
- Coat paper with a foam brush in subdued light. Watercolor or coated inkjet paper works best but any paper will provide an image.
- Let paper dry in a dark place. Image in the sun or source of UV for 10 to 20 minutes. Color of the image should be almost gray or appear over-exposed.
- Rinse image in a couple of changes of water and let dry. Image will intensify as it dries.
|
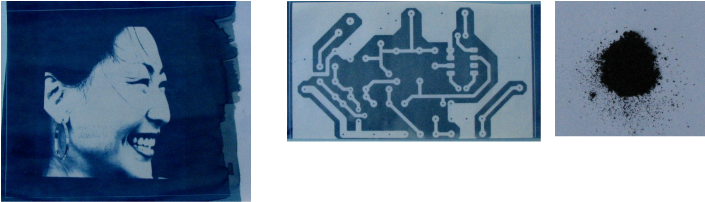
As promised in the last post on photograms, here are my results with real blueprint or cyanotype chemistry. Photo 1 was made using a high contrast negative on inkjet transparency. Photo 2 is a negative printed circuit image that I had made some time ago. Photo 3 is the dye made from the reaction of the two chemicals used after the mix was exposed to light and the water evaporated. The dye formed is Prussian Blue, sometimes called Turnbulls's Blue. The chemicals needed and the formula are below, and at the end of the post, I will provide a source of the ingredients, and a great reference site: This should provide a good start as to what can be an addictive hobby. It can be a real challenge to get images that are exceptional, but also great fun. As with all chemicals, be careful and locate the MSDS, (material safety data sheets) on both chemicals. A very good site for cyanotype and other photographic processes can be found here. A site that I have used successfully for these chemicals and other photographic chemicals also sells a cyanotype kit. From the description, it appears that you only have to add distilled water and mix. It is less expensive to use the kit if you only want to try out the process. But the other chemicals are available in several different quantities if you want to go further. I have had good service from here. Good luck and send questions in the comments section and I will update the post.
0 Comments
Leave a Reply. |
AuthorThe author has an eclectic background in chemistry, electronics, writing, mental health, and community action...Ken Archives
June 2021
|
 RSS Feed
RSS Feed
