Fortunately, we have Jacques Charles to thank, as he is responsible for discovering the relationship between temperature and pressure in an ideal gas. He did not publish his findings in the 1780’s, but later, in the early 1800’s, Gay-Lussac did attribute the discovery to Charles. In today’s language we know that if the amount of air in a tire remains constant, the pressure will be directly proportional to the temperature. So, if a tire has 35 PSI, (Pounds per Square Inch) at 68 F (20 C), and the temperature drops to 32 F, (0 C), the tire pressure will go down. As a result, depending on the tolerance of the sensor, the warning light and low tire symbol may come on. So, to state the law according to Charles:
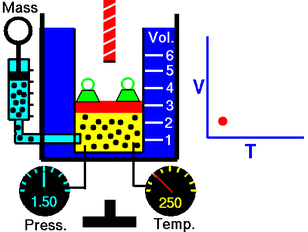
The volume of a given mass of an ideal gas is directly proportional to its temperature (in Kelvin), if pressure and the amount of gas remain constant; that is, the volume of the gas increases or decreases by the same factor as its temperature. This can be seen by the following formula:
V/T = k, where V is the volume of the gas, T is the temperature of the gas, and k, which is a constant. So, if the temperature drops really low in relation to the tire temperature, expect a warning. The best rule to follow is to test tire pressure when the tires are cold, and use a reliable pressure gauge. Fortunately, neither Charles nor Gay-Lussac had to worry about their tires!
 RSS Feed
RSS Feed
